
Electrons — Structure & Properties Expii
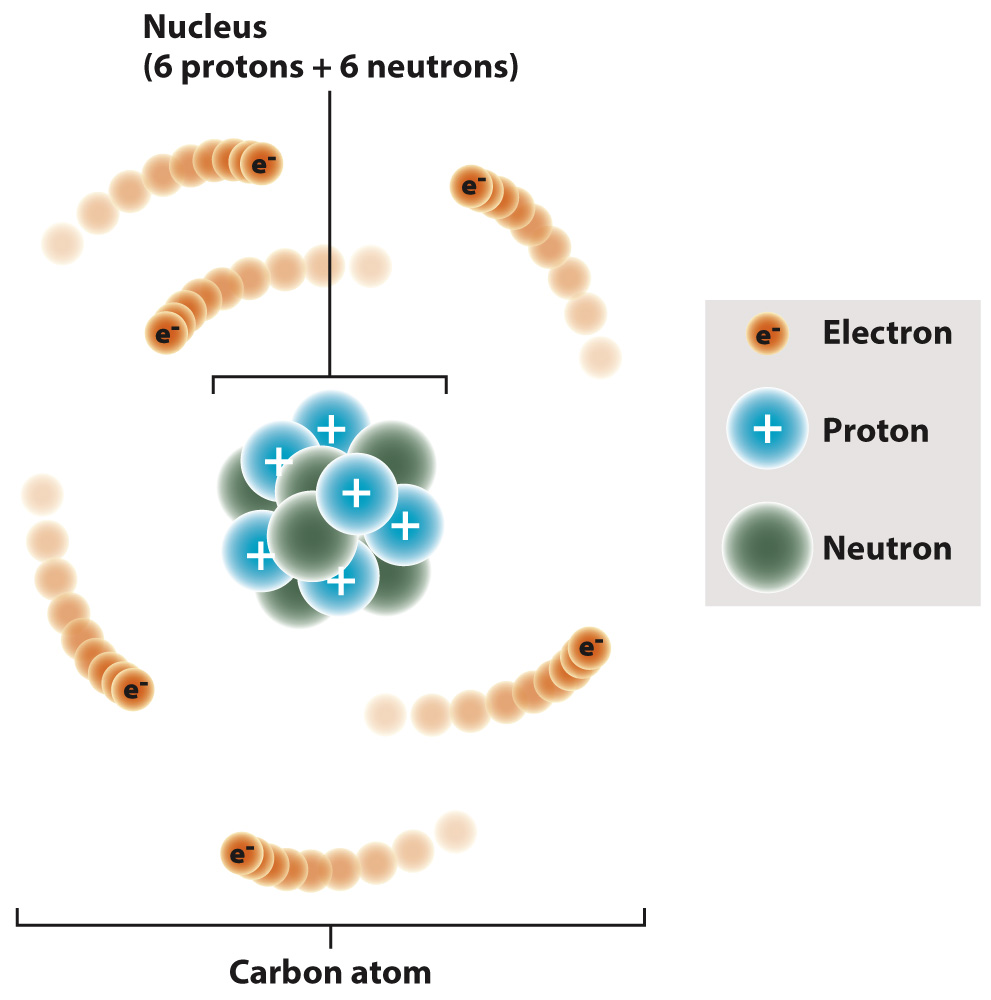
That number is 666. Some people are really very scared of this Biblical passage because of the number 666…we all know why…. However, this has another meaning altogether - it is referring to the subatomic particles that make up carbon: 6 protons, 6 neutrons, 6 electrons. Carbon's atomic number is 6 and it is the 6th most bountiful.
image
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom's mass number is its atomic mass.

Nucleons, Atomic Number and Mass Number Definitions.. and more
Carbon is a chemical element. Its atomic number is 6; its atomic weight is 12.011. It is a group IVA element, residing between boron and nitrogen on the periodic table, and it has 6 protons, 6 neutrons, and 6 electrons.
Periodic Table Of Elements List With Protons Neutrons And Electrons Awesome Home
Study with Quizlet and memorize flashcards containing terms like An atom has 6 protons, 8 neutrons, and 6 electrons. Its atomic mass is:, An atom with 6 protons, 7 neutrons, and 6 electrons shares four pairs of electrons with four other atoms. This atom is now considered to be :, Nucleotides are composed of and more.

Protons neutrons and subatomic particles Pharmacy Gyan
Study with Quizlet and memorize flashcards containing terms like What is the atomic number of an atom that has 6 protons, 6 neutrons, and 6 electrons? 12 0 18 -1 6, Which of these refers to atoms with the same atomic number but different atomic masses? These atoms have different numbers of protons. These atoms have different numbers of electrons. These atoms are isomers. These atoms are.
Periodic Table Of Elements List With Protons Neutrons And Electrons Awesome Home
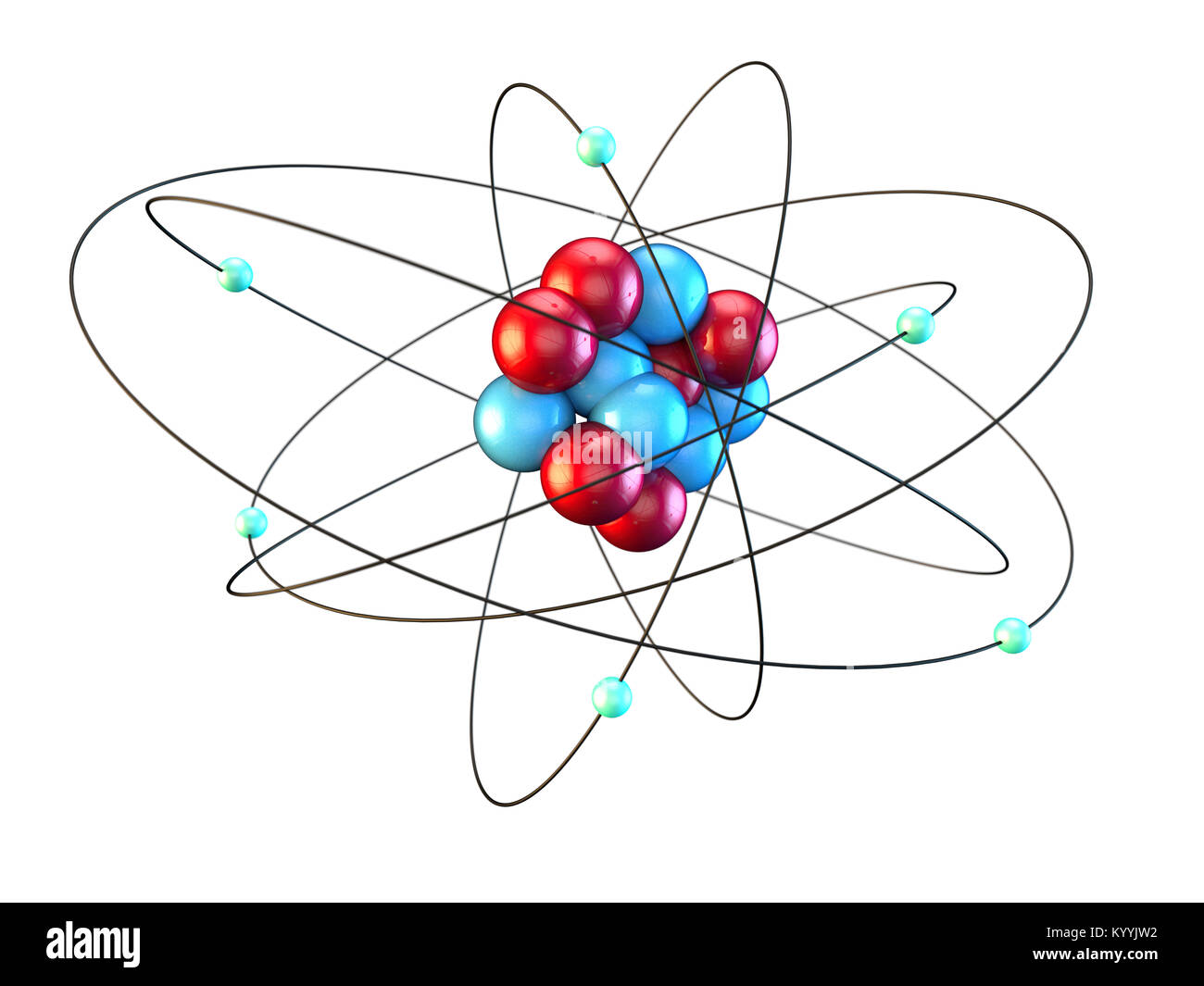
Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus. Neutral atoms have equal numbers of protons and.
Carbon atom showing six electrons orbiting six protons and six neutrons, chemistry chemical
The reduced graphene oxide connection with 3, 4 and 5G and the higher densities from graphene domes or death towers (1000 domes or towers minimum are now operational in every city around the World) are sourced with lasers that have been stripped of their regular electromagnetic waves, leaving only high density magnetic quantum percolation capable of atom to atom interconnects.

666 = The Beast/ Carbon Body 6 Protons, 6 Neutrons, 6 Electrons Spirit science, Physics and
It has an atomic number of 6. That means a carbon atom has 6 protons, 6 neutrons, and 6 electrons. Since carbon is in the second row (or second period), it has 2 electron orbits. Use the clay to make your protons and neutrons in the nucleus. The nucleus should be about the size of a large gumball. (Use two different colors of clay.)
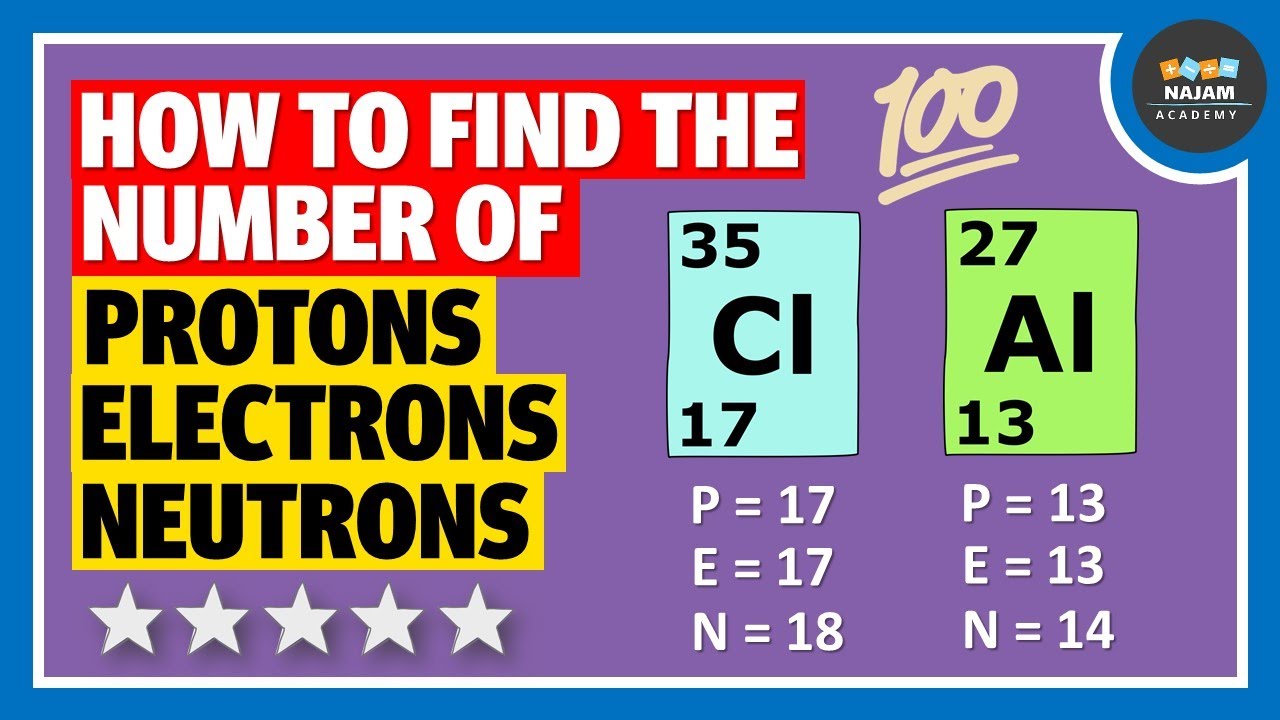
How to find the number of Protons, Neutrons and Electrons? Chemistry YouTube
It is, rather, the number of protons in the nucleus, which we call the atomic number and denote by the symbol Z. Each proton carries an electric charge of +1, so the atomic number also specifies the electric charge of the nucleus. In the neutral atom, the Z protons within the nucleus are balanced by Z electrons outside it.

Periodic Table Carbon Protons Neutrons Electrons Periodic Table Timeline
Lithium has 3 protons, 4 neutrons and 3 electrons. 4. Beryllium has 4 protons, 5 neutrons and 4 electrons. 5. Boron has 5 protons, 6 neutrons and 5 electrons. 6.

Human soul image by Monica Mitchell on (ಠ_ರೃ) Knowlédgé Protons, Electrons
Flexi Says: Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons.

Het aantal neutronen, protonen en elektronen bepalen wikiHow
Study with Quizlet and memorize flashcards containing terms like What is the atomic number of an atom that has 6 protons, 6 neutrons, and 6 electrons? 12 6 18 0 -1, Which of these refers to atoms with the same atomic number but different atomic masses? These atoms have different numbers of protons. These atoms have different numbers of electrons. These atoms are different elements. These atoms.

Atoms & Molecules echapter — The Biology Primer
Explanation: atomic number =number of protons. Therefore it is 6. Answer link. The atomic number is 6, for carbon. The atomic number is equal to the number of protons.
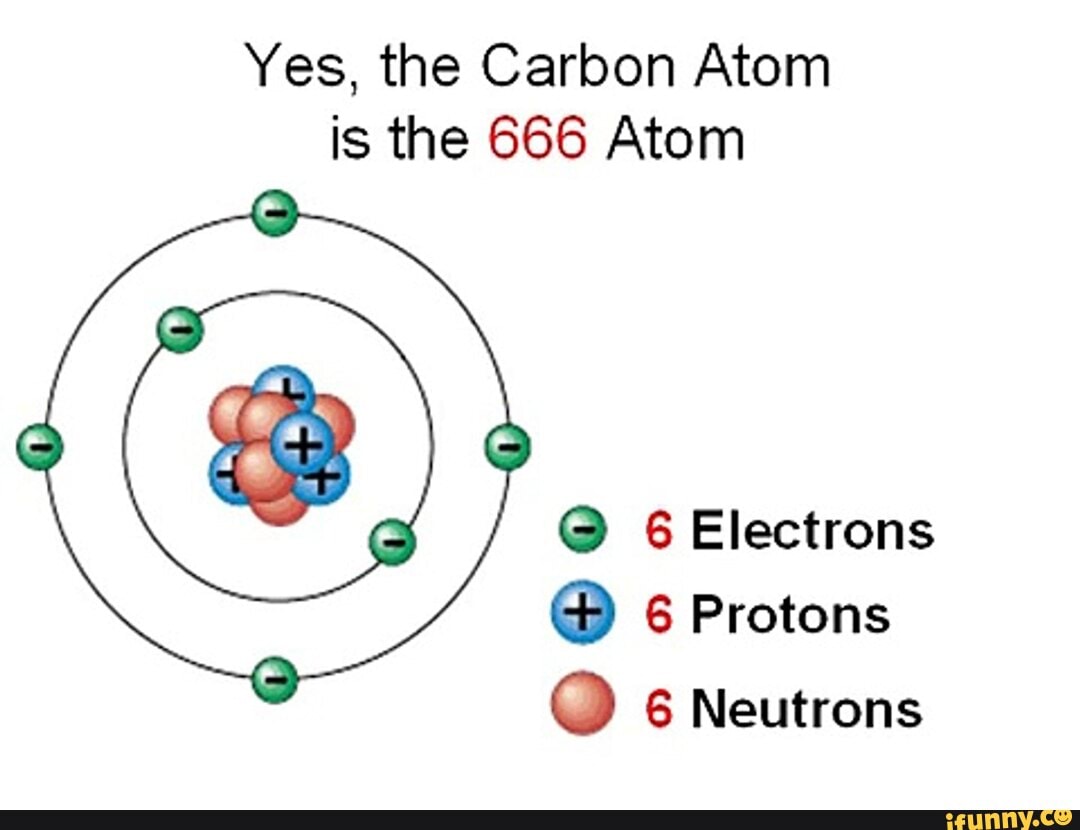
Yes, the Carbon Atom is the 666 Atom 6 Electrons 6 Protons 6 Neutrons iFunny
Each atom has a charged sub-structure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons. The number of protons and the mass number of an atom define the type of atom. Atoms of the same element with different mass numbers are called isotopes. Created by Jay.

Neutron Discovery, Difference and more Teachoo Concepts
The number of protons plus the number of neutrons equals the atomic mass of the element based upon atomic mass units (amus) For this element 6 protons and 6 neutrons combine to make an atomic mass of 12 amus. Lastly, the values of protons and electrons tell whether the atom is an ion or neutral. When protons equal electrons the atom is neutral.
.PNG)
Periodic Table Of Elements Number Of Protons Neutrons And Electrons About Elements
Protons and Neutrons in Carbon. Carbon is a chemical element with atomic number 6 which means there are 6 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.